Affiliated Hospital of Chengdu University
He Bei Yanda Hospital
Youxian Hospital of Chinese Medicine
XuanCheng City Central Hospital
The TCM Hospital of Longquanyi, Chengdu
The People’s Hospital of Danyang
The Affiliated Jiangning Hospital of Nanjing Medical University
Sichuan Tianfu New Area People’s Hospital
Hospital of China Railway No.2 Engineering Group
Anqing First People’s Hospital
World Congress 2023 – Bruxelles, Belgium
24th WORLD STERILIZATION CONGRESS
18th – 21st of OCTOBER 2023
Video of the 2023 Congress in Brussels:
Download Programme:
Download Presentations:
 Lionel Pineau: Comparison of endoscope sampling and culturing methods
Lionel Pineau: Comparison of endoscope sampling and culturing methods
 Matthias Tschoerner: Hygiene safety and resource savings in automated cleaning – A contradiction?
Matthias Tschoerner: Hygiene safety and resource savings in automated cleaning – A contradiction?
 Mads Granlie: Surgical Instrument Tray Optimization and Standardization at AUH
Mads Granlie: Surgical Instrument Tray Optimization and Standardization at AUH
 Mireille Ferlita: Reprocessing Dental Handpieces: A Hope for all and dental Practices?
Mireille Ferlita: Reprocessing Dental Handpieces: A Hope for all and dental Practices?
 Hannah Siwe: Evaluation of a UV-C LED device for disinfection of medical instruments
Hannah Siwe: Evaluation of a UV-C LED device for disinfection of medical instruments
 Randal Eveland: Hospital Sterilization of 3D printed devices
Randal Eveland: Hospital Sterilization of 3D printed devices
 Hugh O’Connor: Global Warming of PCDs
Hugh O’Connor: Global Warming of PCDs
 Xin Zhao: A Study of Establishment and Evaluation of a Risk Prediction Model for Steam Sterilization
Xin Zhao: A Study of Establishment and Evaluation of a Risk Prediction Model for Steam Sterilization
 Jolanda Buijs and Jolyn van der Beek: Agreement on standardised, automated and global sharing of CSSD information
Jolanda Buijs and Jolyn van der Beek: Agreement on standardised, automated and global sharing of CSSD information
 Richard Bancroft: Global Medical Device Standards & Their Acceptability for Regulatory Purposes
Richard Bancroft: Global Medical Device Standards & Their Acceptability for Regulatory Purposes
 Philippe Destrez: ISO 22441 standard for validation of the H2O2 sterilizers explained
Philippe Destrez: ISO 22441 standard for validation of the H2O2 sterilizers explained
 David De Baets: Surgical Instrument Traceability in Sterilization: Legal Obligation vs Necessity?
David De Baets: Surgical Instrument Traceability in Sterilization: Legal Obligation vs Necessity?
 Hamid Zare: Sterilization Risk Management in developing countries
Hamid Zare: Sterilization Risk Management in developing countries
 Roel Beltran Castillo: Mental Health in the department: Lessons Learned from the pandemic
Roel Beltran Castillo: Mental Health in the department: Lessons Learned from the pandemic
Blue Medical B.V.

Blue Medical manufactures and supplies the highest quality sterile barrier systems, sterilization and monitoring products. The assortment ranges from one- and two-phase sterilization wrappings to chemical indicators and instrument protectors. We respond quickly and we treat our worldwide distributors and healthcare professionals as partners. Innovation, low overhead costs and short, cost-efficient supply chains are key factors in our organization. Our company strives for patient safety through sterility. The mission of Blue Medical is to supply a smart and cost-efficient assortment of high quality CSSD-products. Because sterile really matters!
Blue Medical is an ISO 13485:2016 certified company and complies with its products to all latest standards and where appliable the Medical Device Regulation (MDR EU 2017/745).
Do you want to be in touch? Please find our contact details below.
Contact:
Blue Medical B.V.
Sluispolderweg 79
1505 HJ Zaandam
The Netherlands
Web: www.blue-medical.nl
Mail: sales@blue-medical.nl
Fon: +31 75 204 00 93
LinkedIn: www.linkedin.com/company/blue-medical-nl
International Day of Sterilisation Sciences – The General Hospital of Western Theater
International Day of Sterilisation Sciences – Guangyuan Central Hospital
International Day of Sterilisation Sciences – The First Affiliated Hospital of Chengdu Medical College
International Day of Sterilisation Sciences – West China Airport Hospital of Sichuan University
International Day of Sterilisation Sciences – GENERTEC 363 Hospital
International Day of Sterilisation Sciences – Women & Children’s Hospital of Hunan
International Day of Sterilisation Sciences – West China Hospital Sichuan University
International Day of Sterilisation Sciences – Bazhong Central Hospital
International Day of Sterilisation Sciences – Qilu Hospital
International Day of Sterilisation Sciences – Lufeng People’s Hospital
International Day of Sterilisation Sciences – Lushan People’s Hospital
International Day of Sterilisation Sciences – West China Longquan Hospital of Sichuan University
International Day of Sterilisation Sciences – Chengdu Fifth People’s Hospital
International Day of Sterilisation Sciences – The First People’s Hospital of Zhaotong
International Day of Sterilisation Sciences – Affiliated Hospital of Chengdu University
International Day of Sterilisation Sciences – Chengdu No.7 People’s Hospital
International Day of Sterilisation Sciences – Xindu District People’s Hospital of Chengdu
International Day of Sterilisation Sciences – The TCM Hospital of Longquanyi Chengdu
International Day of Sterilisation Sciences – Chengdu Shuangliu Hospital of Chinese Medicine
International Day of Sterilisation Sciences – Chengdu Jinniu People’s Hospital
International Day of Sterilisation Sciences – Sanmenxia Central Hospital
International Day of Sterilisation Sciences – Zhoukou Central Hospital
International Day of Sterilisation Sciences – Xiangcheng People’s Hospital
International Day of Sterilisation Sciences – Xinxiang Central Hospital
International Day of Sterilisation Sciences – ARGENTINA
World Congress 2022 – Barcelona, Spain
23st WORLD STERILIZATION CONGRESS
Date: 16th / 19th NOVEMBER 2022

Congress-Website: www.wfhss-congress.com
Download Programme:
Download Presentations:
Wednesday, 16th Nov. 2022
 Christine Denis: Opening Speech
Christine Denis: Opening Speech
Thursday, 17th Nov. 2022
Session 01:
 Nicolas Lavielle: The physics of sterilization
Nicolas Lavielle: The physics of sterilization
 Nathan Ronsse: Low temperatur steam formaldehyde sterilization
Nathan Ronsse: Low temperatur steam formaldehyde sterilization
Session 02:
 Rodolphe C. Hervé: Endoscopy in the 21st century
Rodolphe C. Hervé: Endoscopy in the 21st century
 Michael Beekes: Alpha-Synuclein Seeds of Parkinson´s Disease…
Michael Beekes: Alpha-Synuclein Seeds of Parkinson´s Disease…
Session 03:
 Frank Daniels: To Borescope Or Not To Borescope
Frank Daniels: To Borescope Or Not To Borescope
Session 04:
 Matthias Buhmann: New insights into chemical passivation of stainless surgical steel…
Matthias Buhmann: New insights into chemical passivation of stainless surgical steel…
 Mathias Tschoerner: Methods for the decontamination of process chemical residues…
Mathias Tschoerner: Methods for the decontamination of process chemical residues…
 Karen Seekamp-Schnieder: Statistical evaluation of protein levels of test objects…
Karen Seekamp-Schnieder: Statistical evaluation of protein levels of test objects…
Friday, 18th Nov. 2022
Session 05:
 Lionel Pineau: Endoscope reprocessing: Retrospective analysis of 90311 samples
Lionel Pineau: Endoscope reprocessing: Retrospective analysis of 90311 samples
 William Leiva: Assessment of novel antimicrobial material to prevent biofilm formation…
William Leiva: Assessment of novel antimicrobial material to prevent biofilm formation…
Session 06:
 Francesco Tessarolo: Monitoring steam penetration in channeled instruments…
Francesco Tessarolo: Monitoring steam penetration in channeled instruments…
Session 07:
 François Barbier: Individual surgical instrument traceability…
François Barbier: Individual surgical instrument traceability…
 Olivier Willieme: Modeling a tool for planning a new CSSD
Olivier Willieme: Modeling a tool for planning a new CSSD
Saturday, 19th Nov. 2022
Session 08:
 Terra Kremer: The Impact of Time and Environmental Conditions on Contaminated Instrumentation
Terra Kremer: The Impact of Time and Environmental Conditions on Contaminated Instrumentation
 Sulisti Holmes: Investigation of the Release of Particles During Phacoemulsification Procedures
Sulisti Holmes: Investigation of the Release of Particles During Phacoemulsification Procedures
Session 09:
 Mayra Samara Ordoñez Díaz: Sustainable development in sterilization departments: A first approach
Mayra Samara Ordoñez Díaz: Sustainable development in sterilization departments: A first approach
 Mercedes García Haro: Safety within the RUMED Debunking myths (Spanish)
Mercedes García Haro: Safety within the RUMED Debunking myths (Spanish)
International Day of Sterilisation Sciences – Hospital Nacional Daniel Alcides Carrión Callao – PERU
International Day of Sterilisation Sciences – No. 989 Hospital
International Day of Sterilisation Sciences – Central Sterile Supply Department of The People´ s Hospital of Wenshan Prefecture
GEISTER Medizintechnik GmbH

Excellence is the result of caring more than others think is wise, risking more than others think is safe, dreaming more than others think is practical, and expecting more than others think is possible.
GEISTER MEDIZINTECHNIK GMBH – founded in 1984 – is a manufacturer and system supplier of surgical instruments for conventional open and minimal invasive (keyhole) surgery with focus on cardiovascular, neuro-, joint and powered surgery.
This outstanding expertise in surgical instruments will be complemented by own solutions for sterilization systems.
Located in Tuttlingen in Southwest-Germany – famous capitol of the surgical industry worldwide. We are dedicated to servicing and supplying the surgical world with the highest quality instruments according to the specific requirements and helping the medical professionals with their patient care in every part in the world. Quality with best German workmanship is a global commitment for us. Our aim is to develop technical innovations for evolving, more patient friendly minimally invasive surgical procedures that yet can maintain or even exceed the long term results of proven conventional procedures.
Contact:
GEISTER® Medizintechnik GmbH
Foehrenstrasse 2
D-78532 Tuttlingen
Germany
Web: www.geister.com
Fon: +49 (0) 7461 / 966 24-0
Fax: +49 (0) 7461 / 966 24-22
Hupfer

Your partner for innovative sterile supplies logistics
At Hupfer we develop our products and systems based on a deep understanding of the logistical structures and individual requirements of clinics and medical departments.
As specialists for sterile supplies logistics, we offer integrated solutions for supplying and disposing of sterile goods. Whether sorting, packaging, organising, transporting, storing or distributing – Hupfer helps you optimize work processes, increase occupational safety and guarantee the highest standards of hygiene for cleaning, disinfecting and sterilising.
With decades of experience, in-depth knowledge of the industry and convincing ideas, we support planners, specialist dealers and users, so that everyone involved can be sure that they can ultimately achieve the goals we all share: safety, cost-effectiveness and genuinely satisfied customers, including the patients.
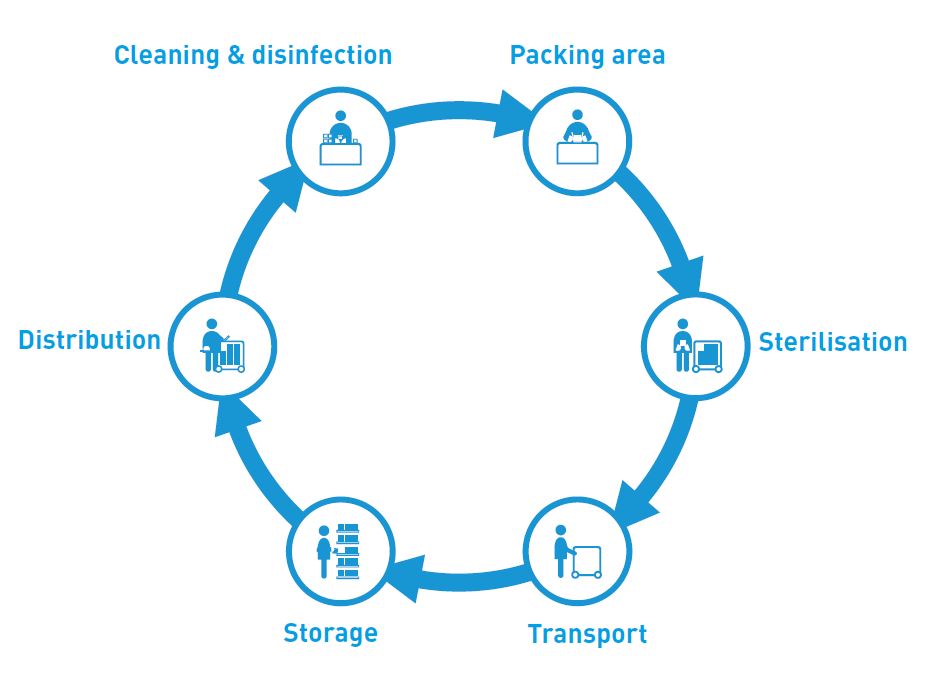
Contact:
HUPFER® Metallwerke GmbH & Co. KG
Dieselstrasse 20
D-48653 Coesfeld
Web: www.hupfer.com
Email: mda@hupfer.org
Fon: +49 2541/805- 370
Fax: +49 2541/805- 379
Mobile: +491704406834
President Dr. Christine Denis speaks about the International Day of Sterilisation Sciences 2021
International Day of Sterilisation Sciences 2021 in TURKEY
International Day of Sterilisation Sciences 2021 in CHINA
 CSSD of The First People’s Hospital Of Jinzhong
CSSD of The First People’s Hospital Of Jinzhong
 CSSD of TEDA INTERNATIONAL CARDIOVASCULAR HOSPITAL
CSSD of TEDA INTERNATIONAL CARDIOVASCULAR HOSPITAL
 CSSD of No.988 Hospital of Joint Logistics Support Force
CSSD of No.988 Hospital of Joint Logistics Support Force
 International Day of Sterilisation Sciences, Xinxiang
International Day of Sterilisation Sciences, Xinxiang
 CSSD of Nanjing Drum Tower Hospital
CSSD of Nanjing Drum Tower Hospital
 CSSD of Luoyang Central Hospital
CSSD of Luoyang Central Hospital
 First Affiliated Hospital of Harbin Medical University
First Affiliated Hospital of Harbin Medical University
 CSSD of Children’s Hospital of Nanjing Medical University
CSSD of Children’s Hospital of Nanjing Medical University
 Scientific Disinfection and Sterilization Secure Safety, Xinxiang Central Hospital
Scientific Disinfection and Sterilization Secure Safety, Xinxiang Central Hospital
 The First Affiliated Hospital of Zhengzhou University
The First Affiliated Hospital of Zhengzhou University
 Zhengzhou University No.3 Affiliated Hospital
Zhengzhou University No.3 Affiliated Hospital
 General Hospital of Western Theater Command
General Hospital of Western Theater Command
 First People’s Hospital of Loudi
First People’s Hospital of Loudi
 Lianyungang Municipal Oriental Hospital
Lianyungang Municipal Oriental Hospital
 Qinghai University Affiliated Hospital
Qinghai University Affiliated Hospital
 Suzhou First People’s Hospital
Suzhou First People’s Hospital
 The First People’s Hospital of Lianyungang
The First People’s Hospital of Lianyungang
Video: International Day of Sterilisation Sciences, Xinxiang
Video: International Day of Sterilisation Sciences, First Affiliated Hospital of Harbin Medical University
Video: Zhengzhou University No.3 Affiliated Hospital
Video: International Day of Sterilisation Sciences of CSSD, Luoyang Central Hospital
Video: International Day of Sterilisation Sciences, The First Affiliated Hospital of Zhengzhou University
Video: International Day of Sterilisation Sciences, General Hospital of Western Theater Command
Video: Suzhou First People’s Hospital
Video: The First Bethune Hospital of Jilin University
Video: The First People’s Hospital of Lianyungang
Video: Xiangyang Central Hospital
Video: The Affiliated Tumor Hospital of Harbin Medical University
International Day of Sterilisation Sciences 2021 in PERÚ
International Day of Sterilisation Sciences 2021 in COLUMBIA
3M Health Care, Medical Solutions Division

3M, with newly-acquired KCI, focuses on providing better care through patient-centered science. Helping transform outcomes by stewarding skin, reducing the risk of preventable complications and restoring lives. From wound and skin care to solutions for BSI and SSI risk reduction, our team is ready to partner with you to strive toward a world with zero complications.
Contact:
3M Health Care, Medical Solutions Division
(Joanna David | SA&E Associate, Strategic Medical Partnerships, EMEA)
Citywest Business Campus, 2050 Orchard Avenue
Dublin 24 | Ireland
Tel: +353 86 467 1270
E-Mail: jdavid2@mmm.com
Web: www.3m.com/KCI






























