World Sterilization Congress 2026

The 27th edition of the World Sterilization Congress takes place on 21st to 24th of October 2026 in Serbia.
More information will follow soon, but make sure you mark your calendar, since it is going to be an amazing chance for you to get updated on the newest insights in the world of the CSSD’s. It is also a great opportunity to meet old friends and make new friends and thus build your network!
Congress website:
wfhss-congress.com
World Sterilization Congress 2025
The 26th edition of the World Sterilization Congress took place on 3rd to 6th December 2025 in Hong Kong.
The theme of the 2025 World Sterilization Congress was “Bring the Sterilization Science to the next level”.
Download Presentations (English / Chinese)

Candidacy for Organization of 2027 WFHSS Annual Congress
It is time to launch the procedure to select the host country for our Annual Congress due to be held in 2027.
An Invitation letter will be published soon…
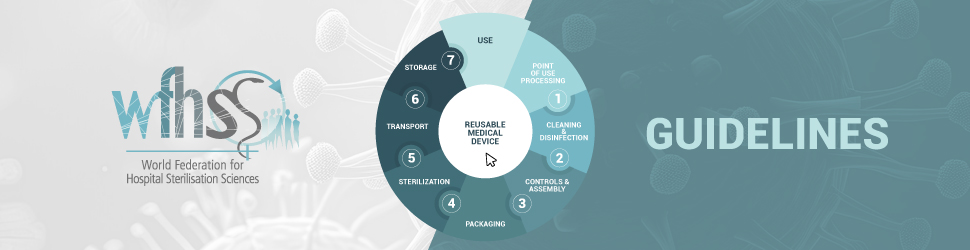
 Guidelines for the Organization of the WFHSS Annual Congress
Guidelines for the Organization of the WFHSS Annual Congress
Video WFHSS Members
Check out our video, showing all WFHSS member countries!